The Oxford/AstraZeneca Covid vaccine has been approved for UK use - what you need to know
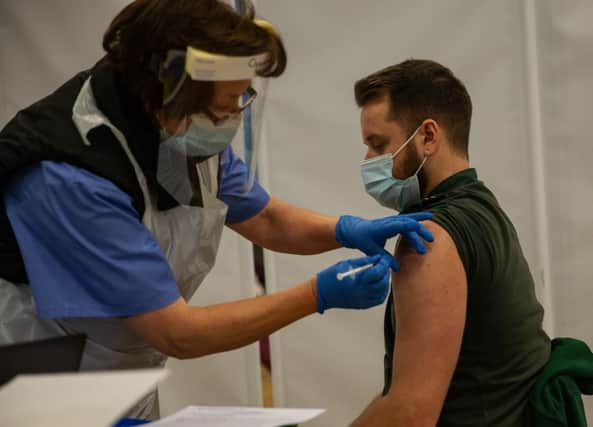

The Oxford/AstraZeneca vaccine has officially been approved for use in the UK, with the first doses set to be administered on Monday 4 January.
The UK has ordered 100 million doses of the vaccine, which is enough to vaccinate 50 million people, as each recipient requires two injections.
Advertisement
Hide AdAdvertisement
Hide AdThe Oxford/AstraZeneca vaccine is also able to be stored in a standard fridge, unlike the Pfizer/BioNTech injection, which requires cold storage of around minus 70C. This will allow the Oxford vaccine to be easier to rollout to places such as care homes and GP surgeries.
‘Passed rigorous clinical trials’
A spokesperson for the Department of Health and Social Care (DHSC) said, “The government has today accepted the recommendation from the Medicines and Healthcare products Regulatory Agency (MHRA) to authorise Oxford University/AstraZeneca’s Covid-19 vaccine for use.
“This follows rigorous clinical trials and a thorough analysis of the data by experts at the MHRA, which has concluded that the vaccine has met its strict standards of safety, quality and effectiveness.”
The statement from the DHSC announcing the news said that, from today (30 Dec), “the NHS across the UK will prioritise giving the first dose of the vaccine to those in the most high risk groups.”
Advertisement
Hide AdAdvertisement
Hide Ad‘A way out of the pandemic’
Speaking to Sky News, Health Secretary Matt Hancock said, “I am now, with this approval this morning, highly confident that we can get enough vulnerable people vaccinated by the spring that we can now see the route out of this pandemic.”
Hancock added that there would still be difficult times ahead, but “we also know that there is a route out of this.
“We have all just got to hold our nerve over the weeks to come.”
Prime Minister Boris Johnson tweeted, “It is truly fantastic news - and a triumph for British science - that the @UniofOxford/@AstraZeneca vaccine has been approved for use.
Advertisement
Hide AdAdvertisement
Hide Ad“We will now move to vaccinate as many people as quickly as possible.”
‘A landmark moment’
Professor Andrew Pollard, director of the Oxford Vaccine Group, who led the clinical trial, said, “The regulator’s assessment that this is a safe and effective vaccine is a landmark moment, and an endorsement of the huge effort from a devoted international team of researchers and our dedicated trial participants.
“Though this is just the beginning, we will start to get ahead of the pandemic, protect health and economies when the vulnerable are vaccinated everywhere, as many as soon as possible.”
Pascal Soriot, AstraZeneca boss, said, “Today is an important day for millions of people in the UK who will get access to this new vaccine.
Advertisement
Hide AdAdvertisement
Hide Ad“It has been shown to be effective, well tolerated, simple to administer and is supplied by AstraZeneca at no profit.”
‘90% protection’
Data published in The Lancet medical journal, showed that the Oxford vaccine was 62 per cent effective in preventing Covid-19 amongst a group of 4,440 people given two standard doses of the vaccine, compared with the 4,455 people given a placebo drug.
However, of the 1,367 people given a half first dose of the vaccine and then followed by a full second dose, there was 90 per cent protection against Covid-19 when compared to a control group of 1,374 people.
The Lancet data, which was peer reviewed, set out full results from clinical trials of more than 20,000 people.
Advertisement
Hide AdAdvertisement
Hide AdAmong those given the placebo drug, 10 were admitted to hospital with Covid-19, including two with severe coronavirus which resulted in one death. However, among those receiving the vaccine, there were no hospital admissions or severe Covid-19 cases.